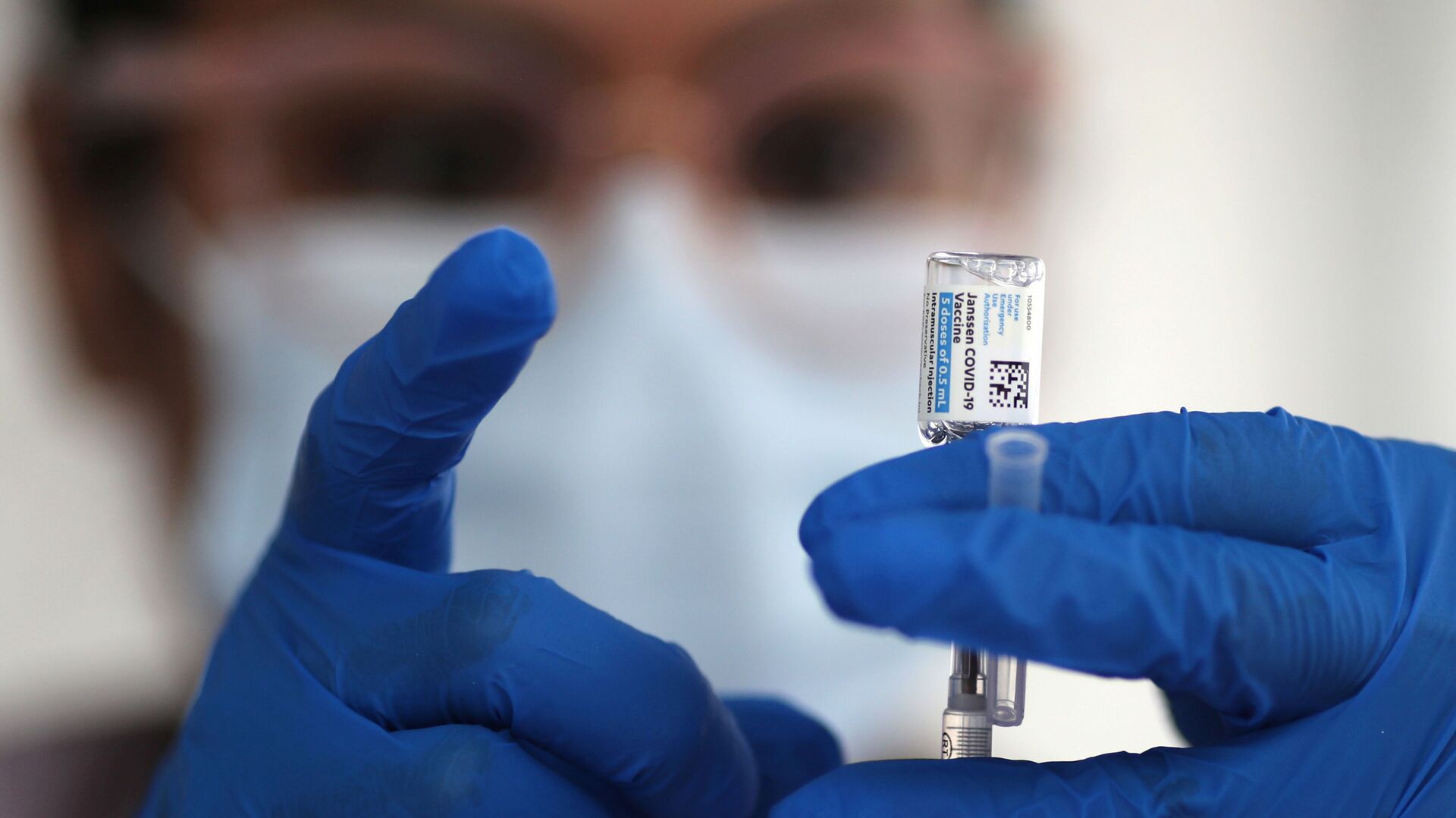
The US federal health agencies are recommending a "pause" in the use of Johnson & Johnson's single-dose COVID-19 vaccine.
In a joint statement, the Centers for Disease Control and Prevention and the Food and Drug Administration announced that the move was taken to allow for an investigation after potentially dangerous blood clots developed in six women in the days after receiving a jab, in conjunction with reduced platelet counts.
— U.S. FDA (@US_FDA) April 13, 2021
As US federal distribution channels, such as mass jab sites, temporarily cease to administer the J&J shot, states and other providers are expected to follow suit.
— U.S. FDA (@US_FDA) April 13, 2021
One patient in the United States has died and another one remains in critical condition due to blood clots after receiving Johnson & Johnson's COVID-19 vaccine, director of the US Food and Drug Administration’s Center for Biologics Evaluation and Research Peter Marks told reporters on Tuesday.
Previously, US health agencies were taking concerns about the Johnson & Johnson Covid-19 vaccine allegedly being linked with a very small increased risk of rare blood clots "seriously", a federal official was cited as saying by CNN.
"The CDC is very concerned and they're very working hard on this and monitoring this closely," said the cited anonymous expert.

Reports of Blood Clots Following Vaccination
This comes as late last week, the European Medicines Agency (EMA) officials cited a probe into three cases of unusual blood clots with low blood platelets following vaccination with the Johnson & Johnson shot, with one more case having occurred in a clinical trial. One of the referenced cases was fatal.
During its April meeting, EMA’s safety committee, the #PRAC, carried out its broad range of responsibilities, which cover all aspects of the risk management of the use of medicines, including #COVID19vaccines. Read more:
— EU Medicines Agency (@EMA_News) April 9, 2021
👉https://t.co/BSurWDTBs4 pic.twitter.com/zFau6OmBuL
The company was collaborating with regulators, and supported "open communication" with healthcare providers regarding any new findings, so that risks might be monitored, said a J&J spokesperson.
The Johnson & Johnson vaccine was rolled out on 3 March, with currently over 6.8 million doses of the jab administered in the US.