The US Food and Drug Administration (FDA) announced on Monday that it would be expanding the emergency use authorization for the Pfizer-BioNTech COVID-19 vaccine to now include adolescents as young as 12.
"The FDA’s expansion of the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include adolescents 12 through 15 years of age is a significant step in the fight against the COVID-19 pandemic," said Dr. Janet Woodcock, the acting FDA commissioner.
"Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic. Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations."
The US health regulator's decision to expand emergency use authorization to include those aged 12 to 15 comes less than two months after Pfizer CEO Albert Bourla declared that he would be sending new data on the Pfizer-BioNTech COVID-19 vaccine to the FDA.
Said data included details from a trial involving 2,260 US adolescents between the ages of 12 and 15 years old. According to the companies, their vaccine "demonstrated 100% efficacy and robust antibody responses, exceeding those recorded earlier in vaccinated participants aged 16 to 25 years old, and was well tolerated."
"It is very important to enable them to get back to everyday school life and to meet friends and family while protecting them and their loved ones," remarked Uğur Şahin, CEO and co-founder of BioNTech.
Additional trials with the Pfizer-BioNTech vaccine have been performed, including those with participants under the age of 11.
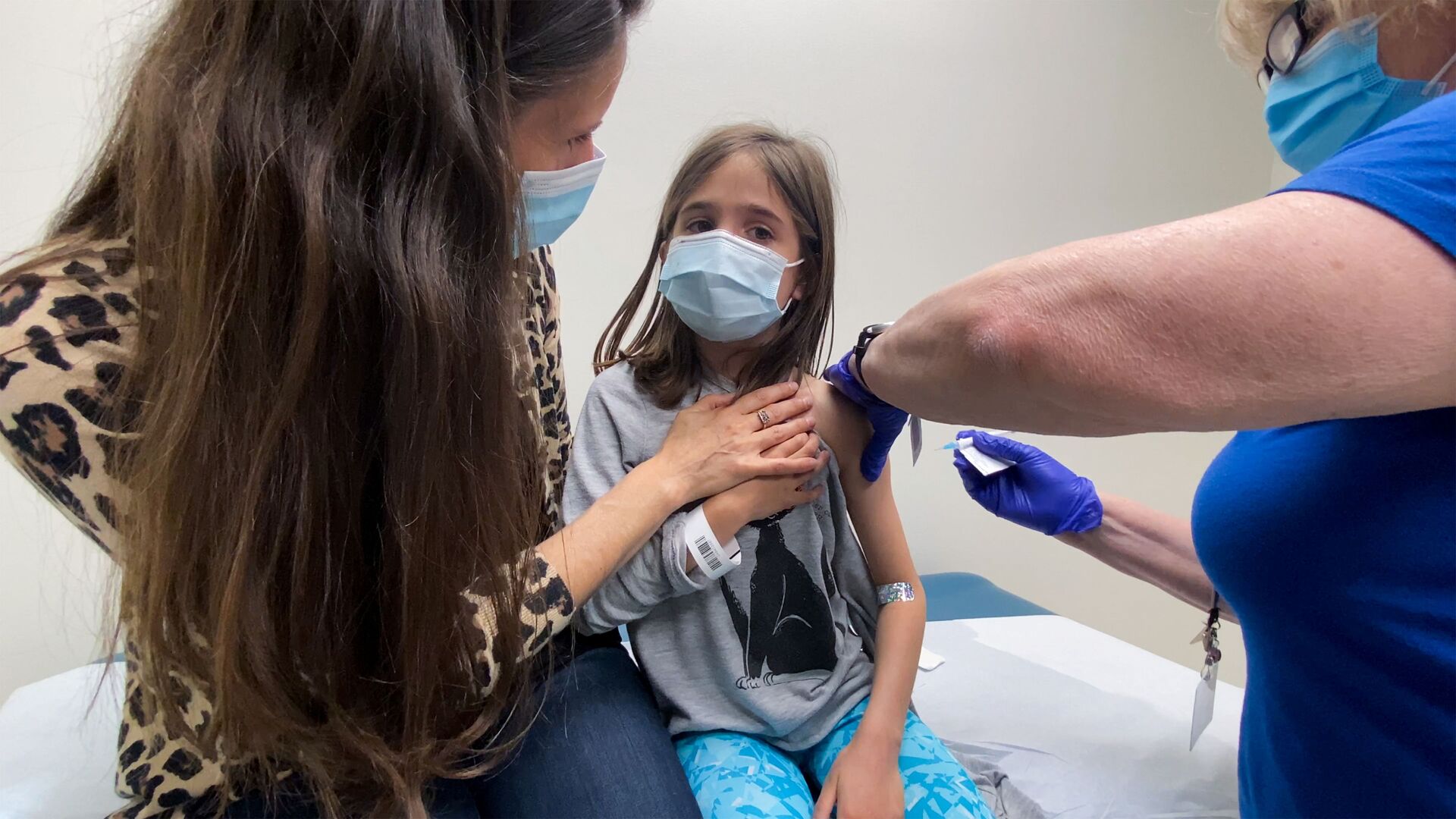
"This is a promising development in our fight against the virus. If you are a parent who wants to protect your child, or a teenager who is interested in getting vaccinated, today's decision is a step closer to that goal," US President Joe Biden said in a Monday statement on the expanded emergency use authorization.
He also highlighted that the US Centers for Disease Control and Prevention (CDC) will issue updated recommendations in the coming days.
As of this article's publication, more than 115 million of the 330 million individuals in the US have been fully vaccinated.
According to data from the CDC, the US has been reporting under 100,000 new COVID-19 cases daily since February 12, and presently has a 7-day moving average of 38,678 new cases a day. As for COVID-19 related deaths, the country has recorded a 7-day moving average of 608 deaths a day.
Experts have suggested that upwards of 80% of the US population must receive COVID-19 vaccines to achieve herd immunity against the contagious disease.