https://sputnikglobe.com/20200615/us-food-and-drug-administration-revokes-emergency-use-of-covid-19-drug-hydroxychloroquine-1079622968.html
US Food and Drug Administration Revokes Emergency Use of COVID-19 Drug Hydroxychloroquine
US Food and Drug Administration Revokes Emergency Use of COVID-19 Drug Hydroxychloroquine
Sputnik International
WASHINGTON (Sputnik) - The US Food and Drug Administration (FDA) said in an alert on Monday that it has revoked the emergency use of hydroxychloroquine, the... 15.06.2020, Sputnik International
2020-06-15T18:26+0000
2020-06-15T18:26+0000
2020-06-15T18:26+0000
https://cdn1.img.sputnikglobe.com/img/107951/23/1079512320_0:148:3073:1886_1920x0_80_0_0_ea5f73cdc779b49b374e073b398df53d.jpg
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
2020
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
News
en_EN
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
us, newsfeed, us food and drug administration, donald trump
us, newsfeed, us food and drug administration, donald trump
US Food and Drug Administration Revokes Emergency Use of COVID-19 Drug Hydroxychloroquine
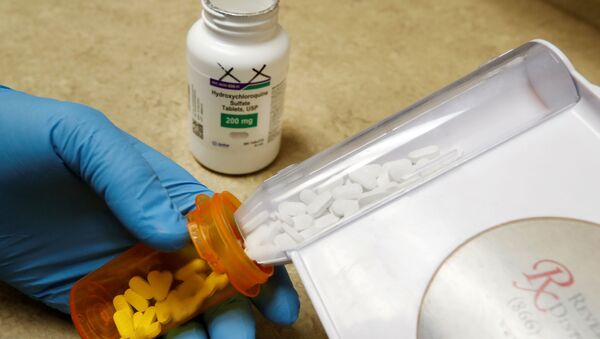
WASHINGTON (Sputnik) - The US Food and Drug Administration (FDA) said in an alert on Monday that it has revoked the emergency use of hydroxychloroquine, the drug touted by President Donald Trump as an effective treatment for the novel coronavirus disease (COVID-19).
“Based on FDA’s continued review of the scientific evidence available for hydroxychloroquine sulfate (HCQ) and chloroquine phosphate (CQ) to treat COVID-19. The FDA has determined that the statutory criteria for EUA as outlined in Section 564(c)(2) of the Food, Drug, and Cosmetic Act are no longer met,” the alert said.
Trump started championing hydroxychloroquine, an anti-malaria drug, as a COVID-19 cure weeks into the pandemic’s outbreak in the United States. The drug has been widely used in France and other countries in combination with zinc and azithromycin to treat COVID-19 patients.
The FDA said in the alert that hydroxychloroquine was not only an ineffective treatment for COVID-19, but was also known to have serious side-effects that could cause cardiac arrest.
“Specifically, FDA has determined that CQ and HCQ are unlikely to be effective in treating COVID-19 for the authorized uses in the EUA,” the alert said. “Additionally, in light of ongoing serious cardiac adverse events and other serious side effects, the known and potential benefits of CQ and HCQ no longer outweigh the known and potential risks for the authorized use.”