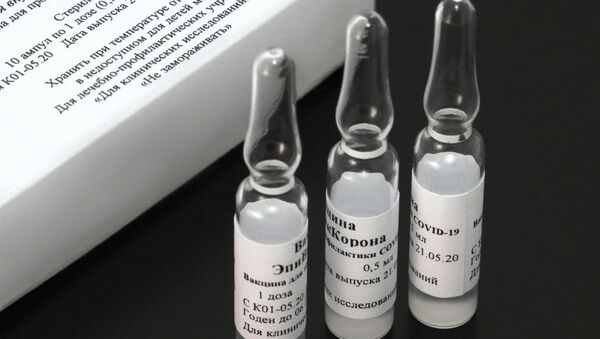
"The peptide antigen-based EpiVacCorona vaccine was [registered] on 13 October and Phase III post-registration trials are currently ongoing", Anna Popova said at an online forum on new scientific data on COVID-19.
In the middle of October, Russia registered its second coronavirus vaccine, EpiVacCorona, which was developed by the Vector research centre.
EpiVacCorona is planned to be introduced into civil circulation on 1 January 2021, according to data from the vaccine registration certificate published in the state registry of medicines.
On 11 August, the country registered the world's first COVID-19 vaccine, Sputnik V, it was developed by the Gamaleya Research Institute with support from the Russian Direct Investment Fund (RDIF).