Despite recent success in securing several million doses of coronavirus vaccines, some European countries are still struggling to vaccinate people with them. But the problem is not the logistics or the lack of jabs – many people in France and Germany are simply reluctant to get a specific shot – the one developed by AstraZeneca, the AFP points out, citing statistics.
The latter shows that France has secured some 1.7 million shots, but only 273,000 of them have been distributed. The situation is no better in Germany, where 240,000 out of 1.45 million AstraZeneca jabs have been administered so far.
France's Attempts to Improve AstraZeneca Shot's Image
The French authorities have tried to dispel concerns about the medicine's efficacy, arising from its uncertain trial results. Head of the MG France doctors' union Jacques Battistoni and vaccination coordinator Alain Fischer slammed attempts to denigrate the quality and efficacy of the AstraZeneca vaccine, calling reports of its inefficacy "deeply unfair". Health Minister Olivier Veran went as far as to take the jab himself in an attempt to prove that it is safe and reliable.
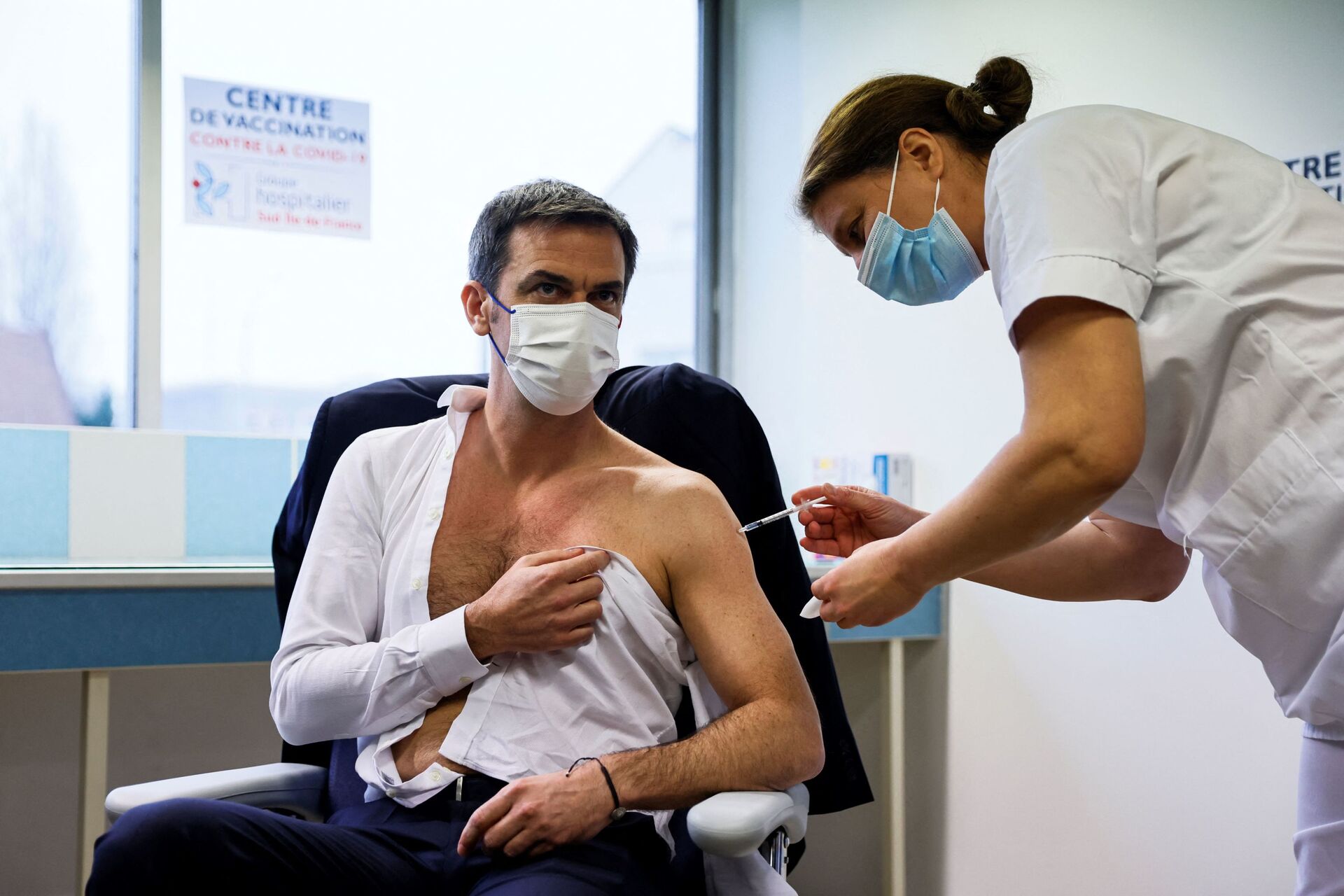
However, the image of the AstraZeneca shot possibly tanked in France before it had even received a green light from the European Medicines Agency (EMA). President Emmanuel Macron alleged that the drug is "quasi-ineffective for people over 65", despite the European regulator approving it for the elderly.
The age limit preventing people over 65 from taking the jab has not helped its popularity either. The vaccination campaign in France is currently also restricted for people who do not have serious health risks, thus hindering the possibility of using unwanted AstraZeneca shots to inoculate other citizens.
Germany Mulls Extending Vaccination Campaign for AstraZeneca Jabs
German Chancellor Angela Merkel tried to boost confidence in the vaccine from the British-Swedish firm by saying that it "can be trusted", but declined to take the jab herself, citing the restriction on giving the shot to people older than 65 – the same as in France.
The unpopularity of AstraZeneca's vaccine among the population currently eligible for immunisation against the coronavirus has prompted calls from local politicians to broaden the scale of the vaccination campaign. Bavarian Prime Minister Markus Soeder argued that no AstraZeneca shots should be lost just because those already eligible for the jabs choose not to take a specific medicine.
"Before that happens: vaccinate anyone who wants it. Every day counts", Soeder said in an interview with Bild am Sonntag newspaper.
Soeder's idea was supported by the state of Baden-Wuerttemberg's Minister-President Winfried Kretschmann, who said that unused AstraZeneca shots must be made available to more people, even if they do not qualify to be at the front of the vaccination line.
AstraZeneca's vaccine was approved by the European Medicines Agency, but the publication of its trial results raised some eyebrows, since the effectiveness of the drug varied between 62% and 90%. According to AstraZeneca, this discrepancy was the result of varying approaches towards vaccination – the dosage and the timeframe between the two shots. The World Health Organisation has set the vaccine's official efficacy at 63% on its information page. The company is running additional trials right now, including one where the jab is mixed with Russia's Sputnik V – the first registered coronavirus vaccine in the world and which has been authorised for use in 39 countries around the globe.






