Omicron-Specific Booster Shots by Pfizer, Moderna Approved by FDA, Will be Available After Labor Day
© AP Photo / Charles Krupa
Subscribe
While the Delta variant of COVID-19 was able to get around vaccines in some instances, the later Omicron variant ended, at least temporarily, the dream of ending the pandemic with mass vaccination, as the new variant proved it could easily infect and re-infect those already inoculated.
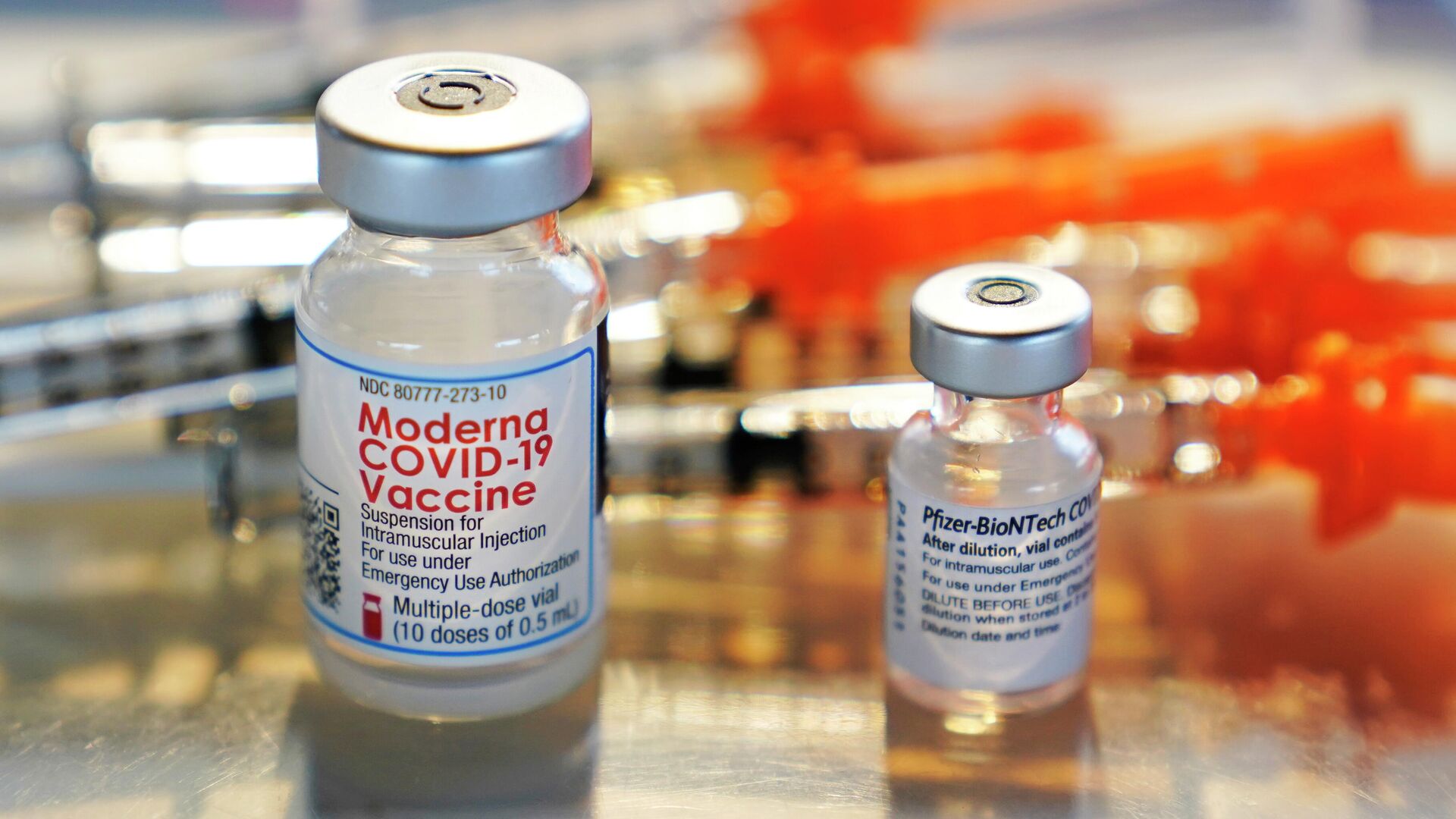
The US Food and Drug Administration (FDA) announced on Wednesday that it had amended the emergency use authorizations for the COVID-19 vaccines produced by Moderna and Pfizer-BioNTech to include “bivalent formulations of the vaccines for use as a single booster dose at least two months following primary or booster vaccination.”
The new formulae, it notes, include two messenger RNA components of SARS-CoV-2, the virus that causes COVID-19: one of the original virus, as the original vaccine had, and the other being a component shared by the BA.4 and BA.5 subvariants of the Omicron variant of the virus, which have emerged within the last year.
The authorization announcement notes that the older, monovalent booster shots are no longer authorized - only these new ones are. However, the monovalent vaccine can still be administered for the initial two-shot vaccination.
Americans over the age of 12 are eligible for the new boosters if they have received either just the base-level vaccination or a booster shot from Moderna, Pfizer-BioNTech, or “any authorized or approved monovalent COVID-19 vaccine.” In the US, that includes the two above, as well as those by Novavax, Johnson & Johnson, AstraZeneca/Oxford, Sinopharm, Sinovac, Covaxin, Covovax, and CanSino.
The FDA notes the new bivalent vaccines “are expected to provide increased protection against the currently circulating Omicron variant,” but it makes no claim about them being able to prevent infection.
“Individuals who receive a bivalent COVID-19 vaccine may experience side effects commonly reported by individuals who receive authorized or approved monovalent mRNA COVID-19 vaccines,” it adds.
“The COVID-19 vaccines, including boosters, continue to save countless lives and prevent the most serious outcomes (hospitalization and death) of COVID-19,” FDA Commissioner Robert Califf said in the news release. “As we head into fall and begin to spend more time indoors, we strongly encourage anyone who is eligible to consider receiving a booster dose with a bivalent COVID-19 vaccine to provide better protection against currently circulating variants.”
Peter Marks, a top vaccine official at the FDA, told the Washington Post that there wasn’t a lot of human-based data to support the boosters, but that waiting until late fall to begin introducing them would have a far worse effect on public health than any problems that might be encountered by administering them sooner.
The FDA’s approval of the two vaccines was based on human studies of older Omicron subvariants, as well as on mouse data. Moderna and Pfizer are both currently performing human-based studies on BA.4 and BA.5, data from which will be available in October.
Marks was optimistic that continuing to roll out booster shots catered to the variants currently circulating “will restore the kind of protections we saw when the vaccines were first launched.”
According to the Post, the new vaccine boosters are expected to become available after the upcoming Labor Day holiday weekend, following a quick review by the US Centers for Disease Control and Prevention (CDC).
The Biden administration has ordered 170 million doses of the boosters, but officials said that only about 10 to 15 million would be available in the first week.
The emergence of the Omicron variant in southern Africa in late 2021 spelled the end of the belief that the pandemic could soon be ended by reaching a critical mass of the population being vaccinated. The new variant, with its numerous evolutions to the spike protein it uses to penetrate a target cell and infect it, proved easily capable of bypassing immune protections generated either by vaccination or by prior infection with COVID-19. It was also much more communicable, spreading much more quickly through populations.
In the months that followed, researchers around the globe raced to develop a new vaccine to account for the latest version of the virus, which continues to kill between 300 and 600 Americans per day, according to CDC data. Meanwhile, the US government has backed off essentially all remaining COVID-19 mitigation practices, including mask and vaccine mandates and limits on indoor activities, even largely removing its ability to track case numbers thanks to a shift toward at-home tests instead of those administered by specialists and reported to health authorities.
Other vaccines that have been modified or reworked to protect against the Omicron variant include the intranasal version of the Gamaleya Institute’s Sputnik V vaccine, and a new mRNA vaccine developed by China National Pharmaceutical Group Corporation, better known as Sinopharm, which was recently submitted for approval by Chinese health authorities prior to beginning trials.