https://sputnikglobe.com/20211020/research-shows-advantages-of-human-adenoviral-vector-jabs-may-give-boost-to-russias-sputnik-v-1090065048.html
Research Shows Advantages of Human Adenoviral Vector Jabs, May Give Boost to Russia's Sputnik V
Research Shows Advantages of Human Adenoviral Vector Jabs, May Give Boost to Russia's Sputnik V
Sputnik International
A group of US scientists pitted several COVID-19 vaccines against each other, with human adenovirus vector jabs outperforming mRNA shots. Such findings are... 20.10.2021, Sputnik International
2021-10-20T11:06+0000
2021-10-20T11:06+0000
2022-08-06T13:32+0000
russia
pfizer
vaccine
astrazeneca
sputnik v vaccine
science & tech
https://cdn1.img.sputnikglobe.com/img/07e5/02/05/1081993272_0:160:3073:1888_1920x0_80_0_0_b0cf0496b557c9a2f237394de1cdaa6c.jpg
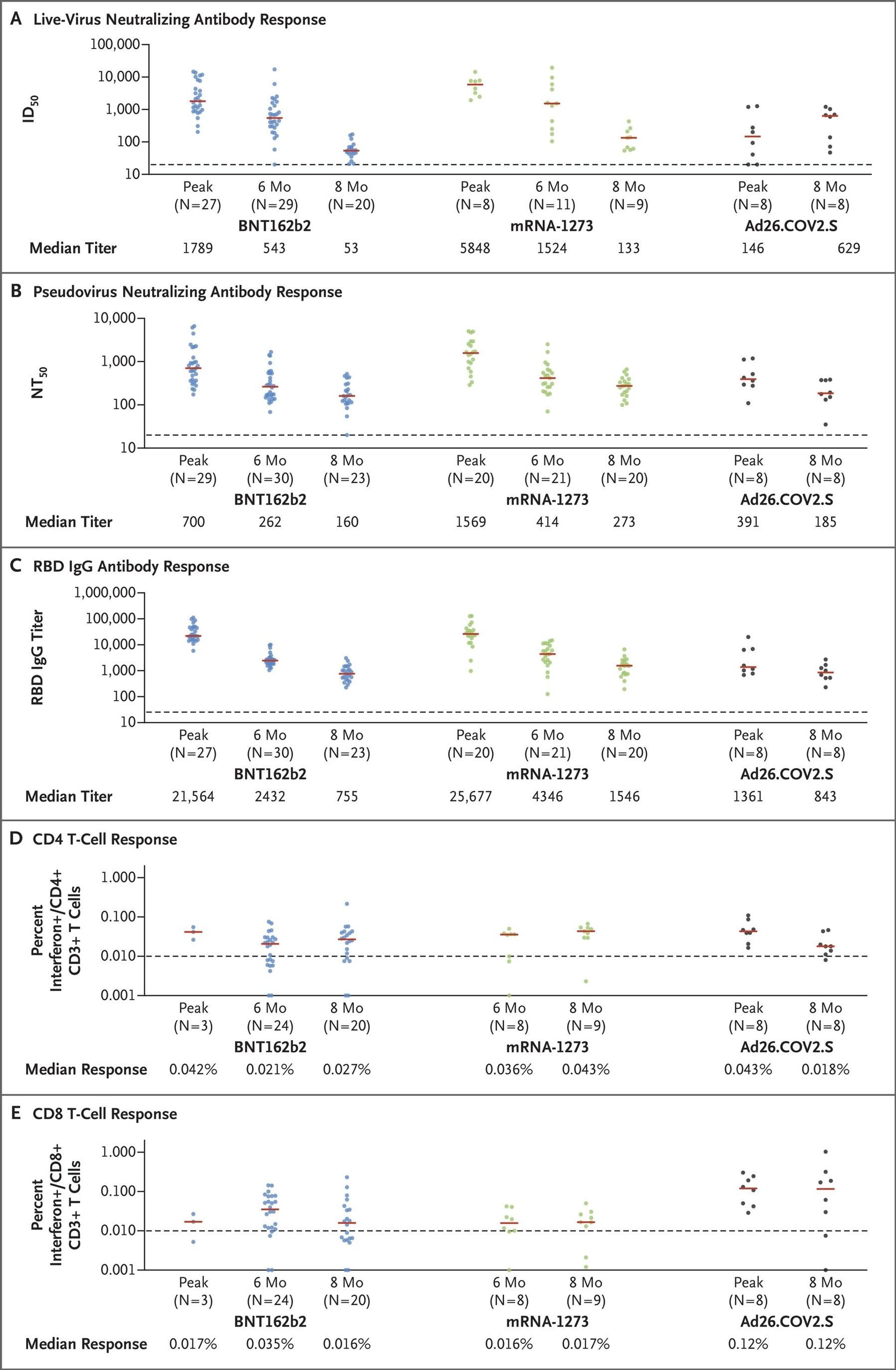
mRNA vaccines provide a high peak antibody response against COVID-19, but lose most of their efficacy within 6 months, while human adenoviral vector jabs provide stable protection levels within 8 months after vaccination - this is the key takeaway of a comparative study published on 15 October in The New England Journal of Medicine.It explored the kinetics of immune responses to the novel coronavirus, induced by the Pfizer, Moderna, and Johnson & Johnson jabs, with the first two vaccines representing mRNA technology, and the third based on the human adenoviral vector platform.The Johnson & Johnson single-shot human adenoviral vector vaccine came out on top of the research, surpassing two of its double-component mRNA competitors, with its live-virus neutralising antibody titer growing from an initial 146 to 629 after 8 months.The number of neutralising protective antibodies for Pfizer's vaccine, which came in second, fell 34-fold after 8 months. The outcome is in line with the data from the 17 September briefing document submitted by Pfizer to the FDA in a bid to secure the administration of booster shots to persons who picked this vaccine brand every 6 months in order to keep the jab's protection level high enough.Moderna's mRNA vaccine came in third, with its antibody response decreasing 44-fold compared to the beginning of an 8-month period.But the level of protective antibodies isn't the only factor that is being taken into consideration by immunologists. The US study also measured the CD8 T-cell response for the two mRNA and one Ad26 vaccine. T-cells are an important part of the immune system that focus on specific foreign particles. T-cell immunity in the case of COVID-19 is actively being researched by scientists all over the world and it's at the forefront of the global effort to thwart the novel coronavirus. This is where the adenoviral vector vaccine won hands down over an 8-month period, with Pfizer eventually showing a CD8 T-cell level of 0.016%, Moderna having a comparable 0.017%, and Janssen (Johnson & Johnson) being at the top with 0.12%.The US study has shown that the initial level of the immune response was quite robust for mRNA vaccines, but it fell to numbers comparable with adenoviral vector jabs after 8 months. The real difference can be seen in the case of the T-cell response – this is where the human adenoviral vector vaccine is the real winner.The results of the American research were echoed by another recent study from Argentina. Argentinian scientists have concluded that Sputnik V, which, just like the Johnson & Johnson vaccine is based on a human adenoviral vector, provides antibody "maturation" and increased protection against the coronavirus within a 6-month period. The Argentinian research showed significant growth in the neutralising potency index (NPI), which was observed at 120 days (NPI=0.33) compared with the values observed at 42 days after vaccination (NPI=0.15).The Pfizer and Moderna vaccines are based on experimental mRNA technology that hasn't been used extensively prior to the COVID-19 pandemic. They utilise a copy of a molecule called "messenger RNA" to produce an immune response. Janssen (Johnson & Johnson) and Sputnik V, for their part, rely on well-known human adenovirus vector carrier technology, where genetic coding for the desired antigen is delivered via a non-replicating virus vector. Both Johnson & Johnson and Sputnik V employ the human adenoviral vector Ad26, but Sputnik V also has a safeguard in the form of a heterologous prime-boost method: if a person has previously been exposed to Ad26 and this component fails to work effectively as a result, the second of the two Sputnik V jabs is designed to bypass this drawback with the use of a different adenoviral platform – Ad5. Both Sputnik V and Janssen use the human adenoviral platform, while AstraZeneca's jab is based on a chimpanzee adenovirus.Sputnik V is the world's first registered COVID-19 vaccine, with over 91.6% efficacy. It is currently authorised for use in 70 countries.
russia
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
2021
Denis Bolotsky
https://cdn1.img.sputnikglobe.com/img/07e5/06/0b/1083128270_0:0:961:960_100x100_80_0_0_8cd81dafcbaac1c176c25141f8af1d2a.jpg
Denis Bolotsky
https://cdn1.img.sputnikglobe.com/img/07e5/06/0b/1083128270_0:0:961:960_100x100_80_0_0_8cd81dafcbaac1c176c25141f8af1d2a.jpg
News
en_EN
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
Denis Bolotsky
https://cdn1.img.sputnikglobe.com/img/07e5/06/0b/1083128270_0:0:961:960_100x100_80_0_0_8cd81dafcbaac1c176c25141f8af1d2a.jpg
russia, pfizer, vaccine, astrazeneca, sputnik v vaccine, science & tech
russia, pfizer, vaccine, astrazeneca, sputnik v vaccine, science & tech
Research Shows Advantages of Human Adenoviral Vector Jabs, May Give Boost to Russia's Sputnik V
11:06 GMT 20.10.2021 (Updated: 13:32 GMT 06.08.2022) A group of US scientists pitted several COVID-19 vaccines against each other, with human adenovirus vector jabs outperforming mRNA shots. Such findings are also backed by Argentinian scientists, who explored long-term data.
mRNA vaccines provide a high peak antibody response against COVID-19, but lose most of their efficacy within 6 months, while human adenoviral vector jabs provide stable protection levels within 8 months after vaccination - this is the
key takeaway of a comparative study published on 15 October in
The New England Journal of Medicine.
It explored the kinetics of immune responses to the novel coronavirus, induced by the Pfizer, Moderna, and Johnson & Johnson jabs, with the first two vaccines representing mRNA technology, and the third based on the human adenoviral vector platform.
The Johnson & Johnson single-shot human adenoviral vector vaccine came out on top of the research, surpassing two of its double-component mRNA competitors, with its live-virus neutralising antibody titer growing from an initial 146 to 629 after 8 months.
The number of neutralising protective antibodies for Pfizer's vaccine, which came in second, fell 34-fold after 8 months. The outcome is in line with the data from the 17 September
briefing document submitted by Pfizer to the FDA in a bid to secure the administration of booster shots to persons who picked this vaccine brand every 6 months in order to keep the jab's protection level high enough.
Moderna's mRNA vaccine came in third, with its antibody response decreasing 44-fold compared to the beginning of an 8-month period.
But the level of protective antibodies isn't the only factor that is being taken into consideration by immunologists. The US study also measured the CD8 T-cell response for the two mRNA and one Ad26 vaccine. T-cells are an important part of the immune system that focus on specific foreign particles. T-cell immunity in the case of COVID-19 is actively being researched by scientists all over the world and it's at the forefront of the global effort to thwart the novel coronavirus.
This is where the adenoviral vector vaccine won hands down over an 8-month period, with Pfizer eventually showing a CD8 T-cell level of 0.016%, Moderna having a comparable 0.017%, and Janssen (Johnson & Johnson) being at the top with 0.12%.
The US study has shown that the initial level of the immune response was quite robust for mRNA vaccines, but it fell to numbers comparable with adenoviral vector jabs after 8 months. The real difference can be seen in the case of the T-cell response – this is where the human adenoviral vector vaccine is the real winner.
The results of the American research were echoed by
another recent study from Argentina. Argentinian scientists have concluded that Sputnik V, which, just like the Johnson & Johnson vaccine is based on a human adenoviral vector, provides antibody "maturation" and increased protection against the coronavirus within a 6-month period. The Argentinian research showed significant growth in the neutralising potency index (NPI), which was observed at 120 days (NPI=0.33) compared with the values observed at 42 days after vaccination (NPI=0.15).
The Pfizer and Moderna vaccines are based on experimental mRNA technology that hasn't been used extensively prior to the COVID-19 pandemic. They utilise a copy of a molecule called "messenger RNA" to produce an immune response. Janssen (Johnson & Johnson) and Sputnik V, for their part, rely on well-known human adenovirus vector carrier technology, where genetic coding for the desired antigen is delivered via a non-replicating virus vector.
Both Johnson & Johnson and Sputnik V employ the human adenoviral vector Ad26, but
Sputnik V also has a safeguard in the form of a heterologous prime-boost method: if a person has previously been exposed to Ad26 and this component fails to work effectively as a result, the second of the two Sputnik V jabs is designed to bypass this drawback with the use of a different adenoviral platform – Ad5. Both Sputnik V and Janssen use the human adenoviral platform, while AstraZeneca's jab is based on a chimpanzee adenovirus.
Sputnik V is the world's
first registered COVID-19 vaccine, with over 91.6% efficacy. It is currently authorised for use in 70 countries.