https://sputnikglobe.com/20211014/us-health-agencies-to-decide-on-approval-for-jj-booster-shot-in-coming-weeks-biden-says-1089930107.html
US Health Agencies to Decide on Approval for J&J Booster Shot in Coming Weeks, Biden Says
US Health Agencies to Decide on Approval for J&J Booster Shot in Coming Weeks, Biden Says
Sputnik International
WASHINGTON, October 14 (Sputnik) - The Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) will decide in the coming... 14.10.2021, Sputnik International
2021-10-14T17:33+0000
2021-10-14T17:33+0000
2021-10-14T17:33+0000
johnson & johnson
vaccines
covid-19
https://cdn1.img.sputnikglobe.com/img/07e5/04/13/1082670475_0:329:3031:2033_1920x0_80_0_0_10cf2254a5c10c8f0c94a1683d07942f.jpg
"We expect a final decision from the FDA and the Centers for Disease Control and Prevention (CDC) in the next couple of weeks," Biden said during a press conference.Biden said the FDA and CDC are currently reviewing data on the vaccine.A study by the US National Institutes of Health showed that a third dose of the Johnson & Johnson COVID-19 vaccine produces fewer antibodies than Pfizer or Moderna boosters.The FDA has so far cleared the Pfizer coronavirus vaccine booster for use in the United States while the other two manufacturers Moderna and Johnson & Johnson are awaiting approval.
https://sputnikglobe.com/20210928/over-400000-americans-receive-covid-19-booster-shot-during-weekend--1089492344.html
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
2021
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
News
en_EN
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
johnson & johnson, vaccines, covid-19
johnson & johnson, vaccines, covid-19
US Health Agencies to Decide on Approval for J&J Booster Shot in Coming Weeks, Biden Says
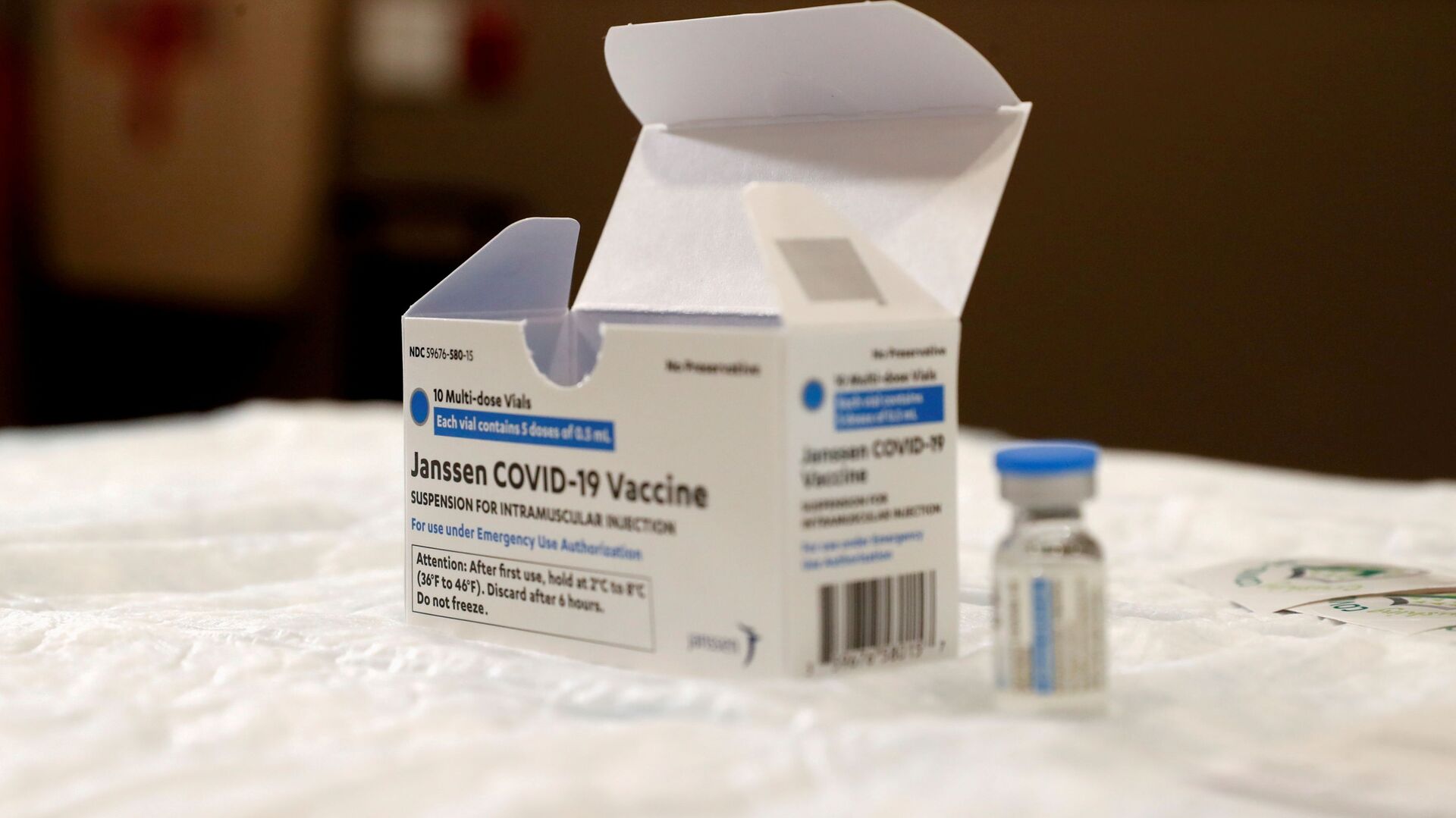
WASHINGTON, October 14 (Sputnik) - The Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) will decide in the coming weeks whether to approve the use of booster shots of the Johnson & Johnson coronavirus vaccine, US President Joe Biden said on Thursday.
"We expect a final decision from the FDA and the Centers for Disease Control and Prevention (CDC) in the next couple of weeks," Biden said during a press conference.
Biden said the FDA and CDC are currently reviewing data on the vaccine.
A study by the US National Institutes of Health showed that a third dose of the Johnson & Johnson COVID-19 vaccine produces fewer antibodies than Pfizer or Moderna boosters.
28 September 2021, 17:43 GMT
The FDA has so far cleared the Pfizer coronavirus vaccine booster for use in the United States while the other two manufacturers Moderna and Johnson & Johnson are awaiting approval.