https://sputnikglobe.com/20210927/pfizer-starts-second-phase-of-testing-new-antiviral-covid-19-oral-drug-in-exposed-adults-1089455819.html
Pfizer Starts Second Phase of Testing New Antiviral COVID-19 Oral Drug in Exposed Adults
Pfizer Starts Second Phase of Testing New Antiviral COVID-19 Oral Drug in Exposed Adults
Sputnik International
WASHINGTON (Sputnik) - Pfizer has started the second phase in the study of its new oral antiviral drug to prevent COVID-19 infection in adults exposed to the... 27.09.2021, Sputnik International
2021-09-27T16:06+0000
2021-09-27T16:06+0000
2021-09-27T16:06+0000
drug
pfizer
vaccine
covid-19
https://cdn1.img.sputnikglobe.com/img/07e5/05/1a/1083003393_0:0:3051:1717_1920x0_80_0_0_206e0debe9cd565d0a7e79d690e50d9b.jpg
"Pfizer Inc. today announced the start of the Phase 2/3 EPIC-PEP (Evaluation of Protease Inhibition for COVID-19 in Post-Exposure Prophylaxis) study to evaluate the investigational novel oral antiviral candidate PF-07321332, co-administered with a low dose of ritonavir, for the prevention of COVID-19 infection," the company said in a release.The new study is enrolling individuals 18 and older living in the same household with an individual who has a confirmed symptomatic coronavirus infection, the release said.If successful, the oral antiviral therapy will be able to stop the virus in the early stages in individuals exposed to the novel coronavirus and prevent the virus from replicating itself and inhibiting the onset of infection in others, Pfizer Chief Scientific Officer Mikael Dolsten said.On Sunday, Pfizer CEO Albert Bourla said the world would be able to return to normal life within a year provided there will be annual revaccinations.
https://sputnikglobe.com/20210923/cdc-panel-endorses-pfizer-booster-shots-for-older-at-risk-americans-1089349745.html
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
2021
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
News
en_EN
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
Sputnik International
feedback@sputniknews.com
+74956456601
MIA „Rosiya Segodnya“
drug, pfizer, vaccine, covid-19
drug, pfizer, vaccine, covid-19
Pfizer Starts Second Phase of Testing New Antiviral COVID-19 Oral Drug in Exposed Adults
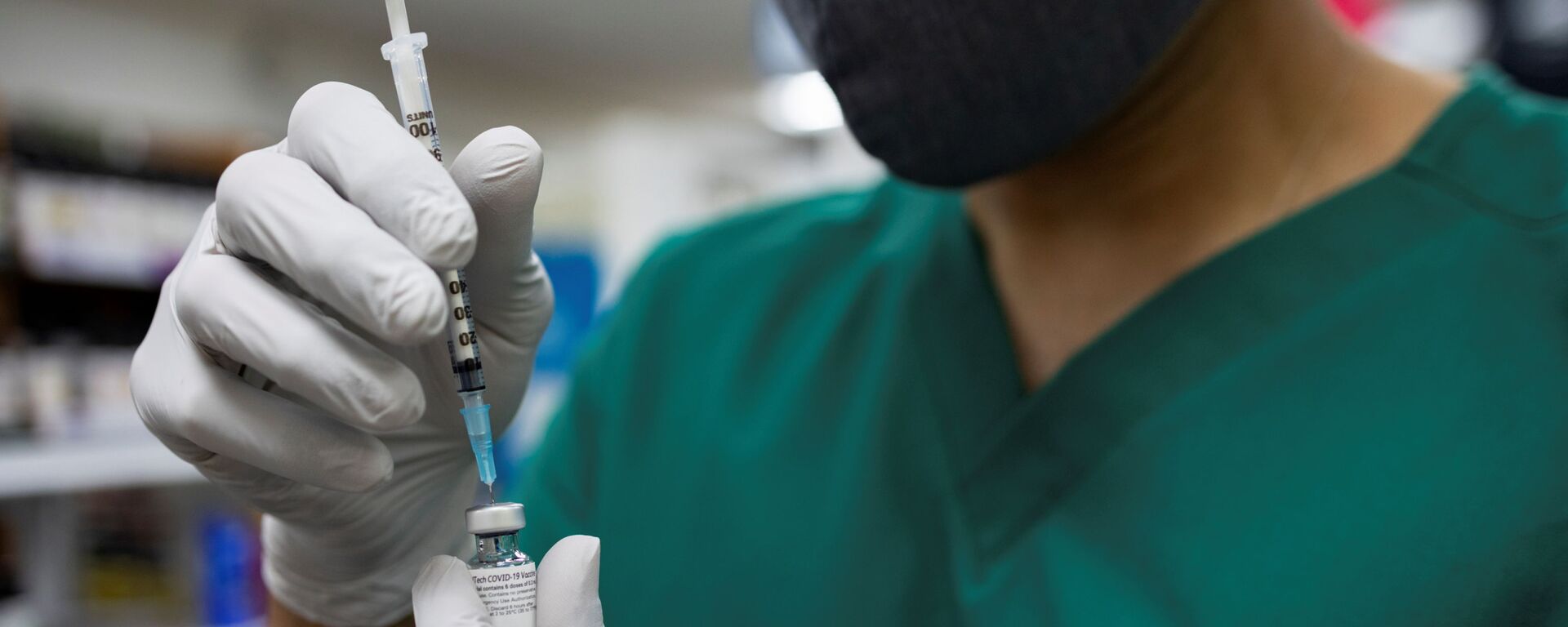
WASHINGTON (Sputnik) - Pfizer has started the second phase in the study of its new oral antiviral drug to prevent COVID-19 infection in adults exposed to the novel coronavirus, the company said on Monday.
"Pfizer Inc. today announced the start of the Phase 2/3 EPIC-PEP (Evaluation of Protease Inhibition for COVID-19 in Post-Exposure Prophylaxis) study to evaluate the investigational novel oral antiviral candidate PF-07321332, co-administered with a low dose of ritonavir, for the prevention of COVID-19 infection," the company said in a release.
The new study is enrolling individuals 18 and older living in the same household with an individual who has a confirmed symptomatic coronavirus infection, the release said.
23 September 2021, 20:33 GMT
If successful, the oral antiviral therapy will be able to stop the virus in the early stages in individuals exposed to the novel coronavirus and prevent the virus from replicating itself and inhibiting the onset of infection in others, Pfizer Chief Scientific Officer Mikael Dolsten said.
On Sunday, Pfizer CEO Albert Bourla said the world would be able to return to normal life within a year provided there will be annual revaccinations.




