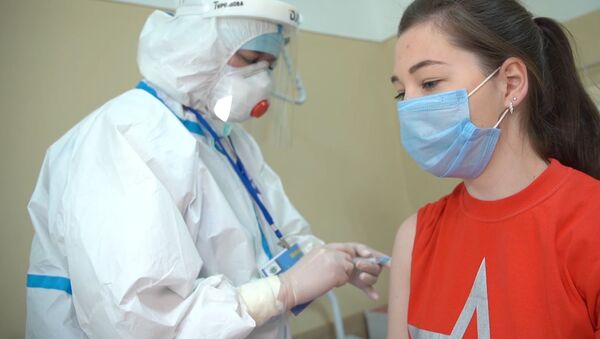
Russian Health Minister Mikhail Murashko said that Russia will launch the large-scale vaccination of people who are especially vulnerable to COVID-19 in November or December.
"For risk groups, the first vaccines will begin to arrive in small quantities as early as September, and more massive vaccination coverage is planned after November-December," the minister said.
Post-registration trial of the COVID-19 vaccine will begin this week, he added.
"This week, post-registration clinical trials begin; groups of volunteers totaling 40 thousand people have been recruited. These are placebo-controlled studies that will allow us to track all the nuances and details, including in a large population. We see that the vaccine is effective and safe," Murashko said.
In August, the Russian government officially registered Sputnik V as the world's first vaccine against COVID-19. Though the vaccine is still underway with the third — last — phase of clinical trials, as per the protocols of the World Health Organization, Russian health officials said it had proven the capability to produce stable immunity against the coronavirus.
Several other countries have followed the lead and announced they would fast-track the registration of their COVID-19 vaccines for emergency use.